What?
We make molecules! Varying from complex natural products to scalable building blocks. Out of curiosity or for application
How?
By careful synthesis design, developing novel synthesis and catalysis methodology, and careful selection of methodology from the literature.
Why?
Because complex molecule synthesis is at the frontiers of modern chemistry, it feeds the development in catalysis, in the life science and in materials science. It offers an excellent training for researchers in molecular sciences.
Research Topic 1
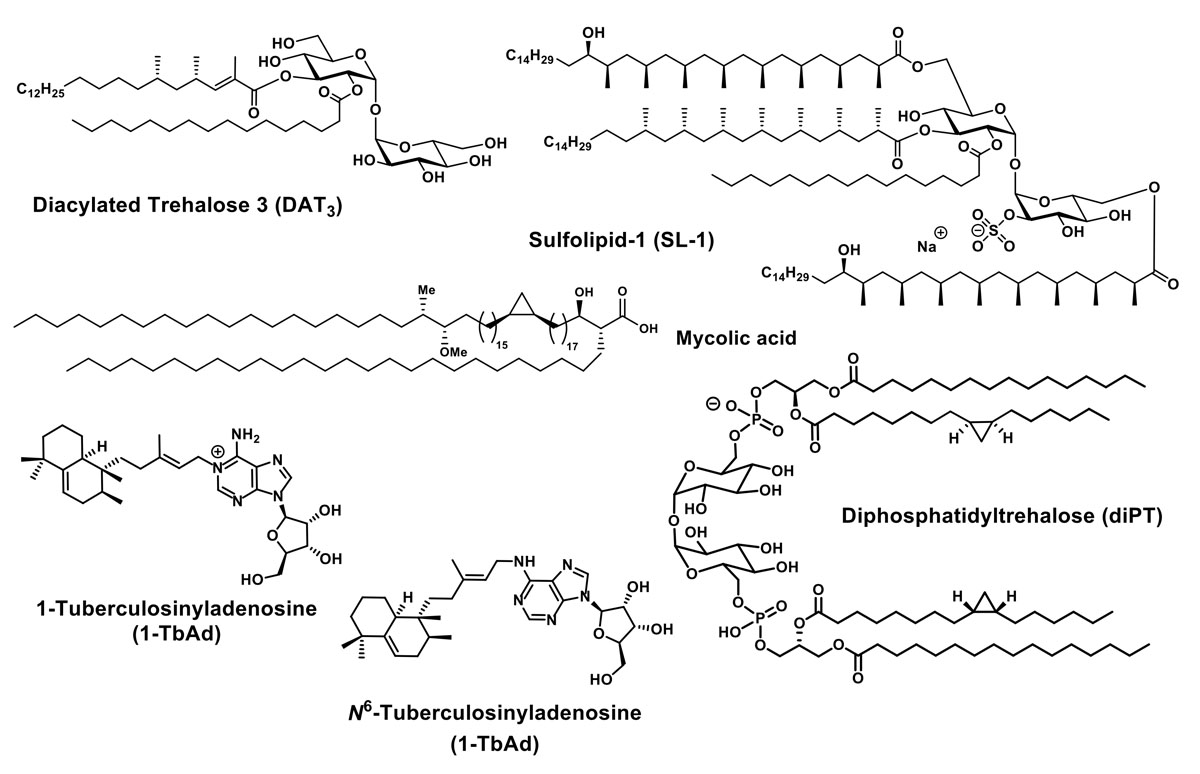
The molecular immunology of Mycobacterium tuberculosis
We study the synthesis and structure elucidation of complex glycolipids found in mycobacteria. Aim is to contribute to the diagnosis of, and vaccine development against, mycobacterial diseases, in particular tuberculosis. We envision that synthetic vaccines in this particular field can elicit a major breakthrough and that diagnostic tests based on small molecules could be very effective in developing countries. The research is carried out in close collaboration with groups in immunology, microbiology and membrane biochemistry.
Research Topic 2
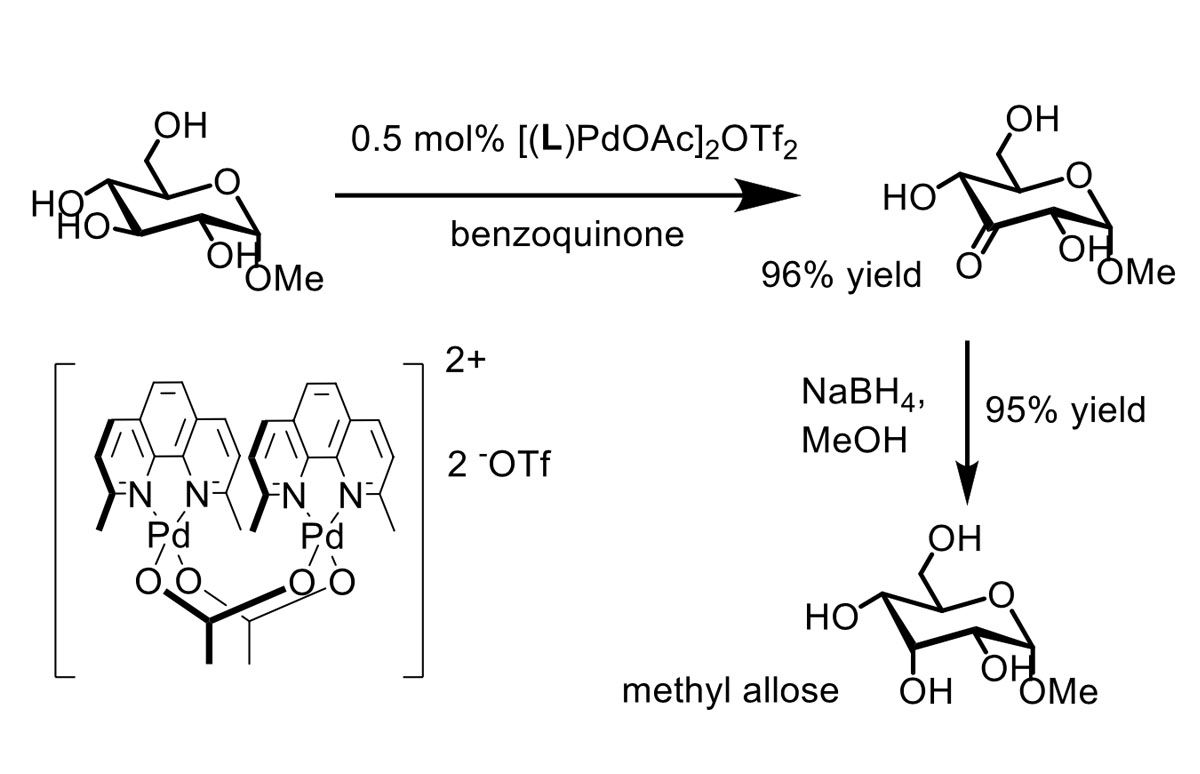
Selective modification of unprotected carbohydrates
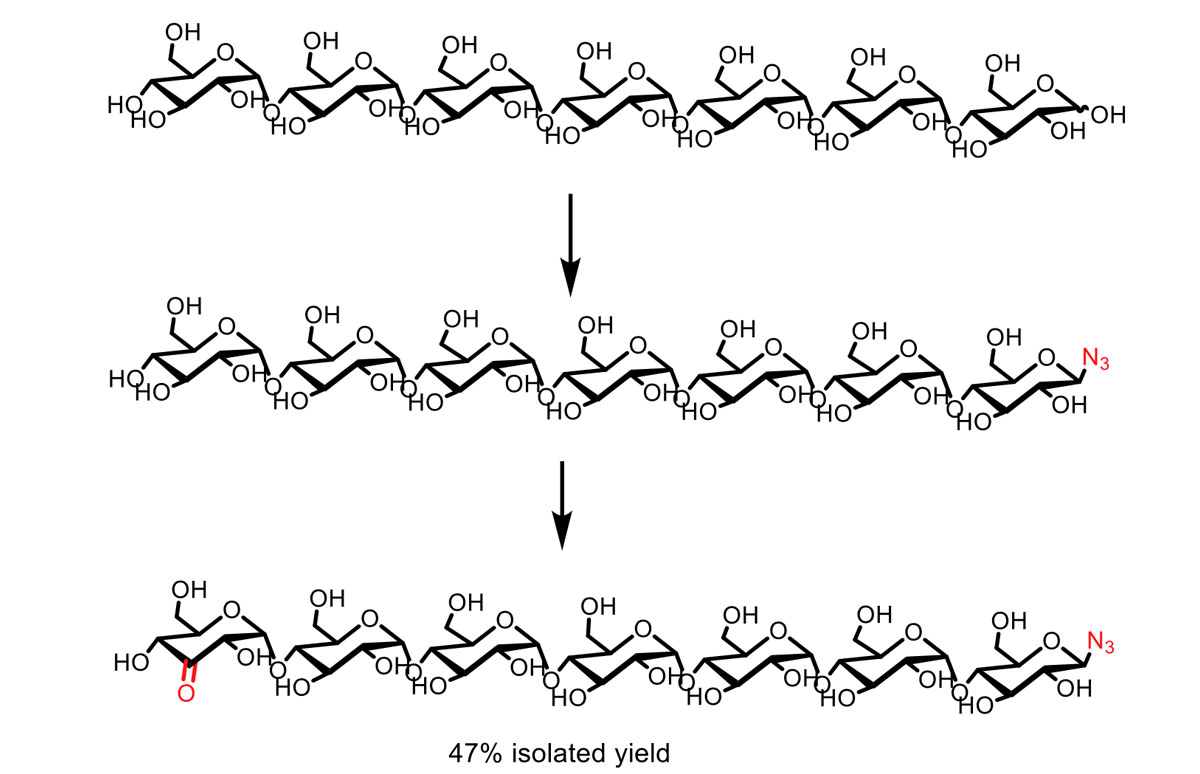
We have shown that palladium-catalyzed alcohol oxidation allows the chemo- and regioselective modification of unprotected carbohydrates, varying from glucose to unprotected 1,4 linked glucans. This is demonstrated in the two-step bisfunctionalization of 1,4 linked glucans up to the 7-mer. Introduction of an anomeric azide is followed by a highly regioselective mono-oxidation of the terminal C3–OH functionality.
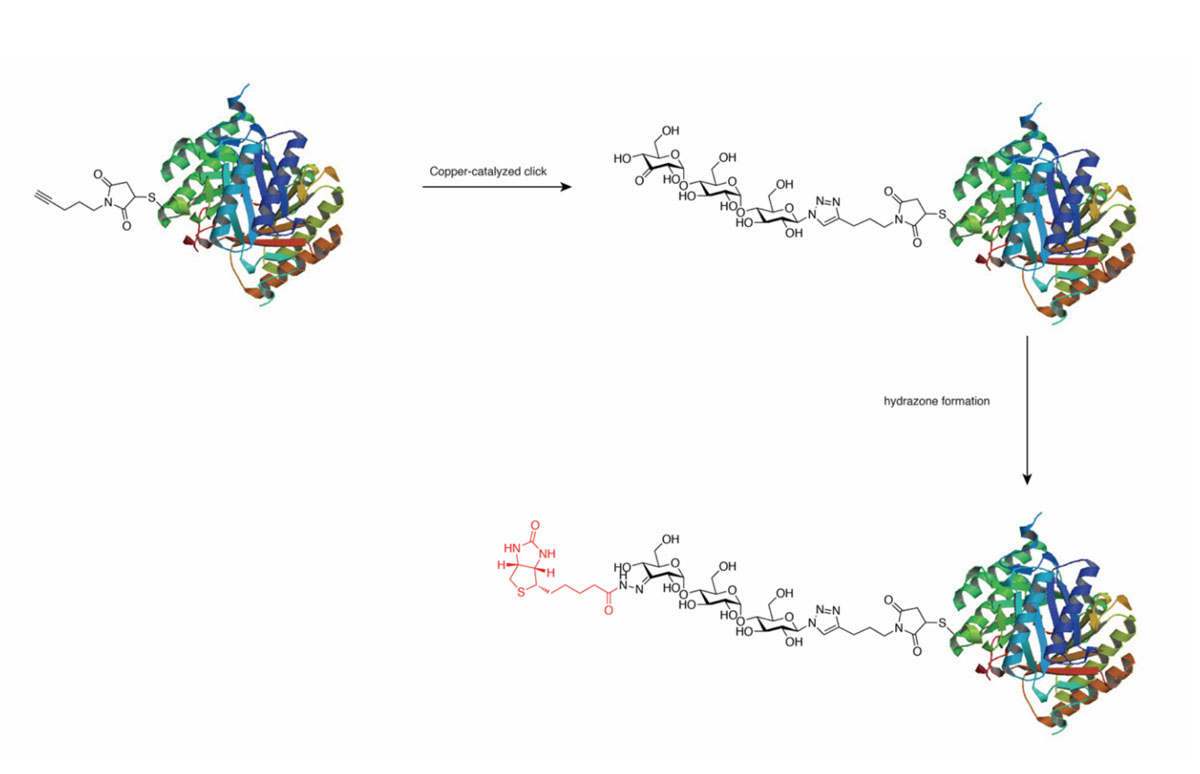
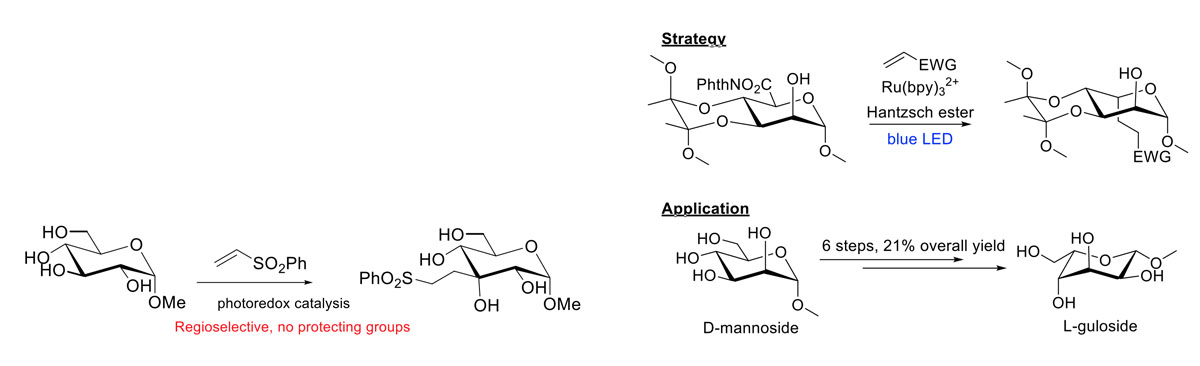
Photocatalysis turns out to be a versatile method for the modification of unprotected carbohydrates. We have used it to selectively place a handle onto monosaccharides and to convert in a couple of steps a D-sugar into an L-sugar!
Research Topic 3
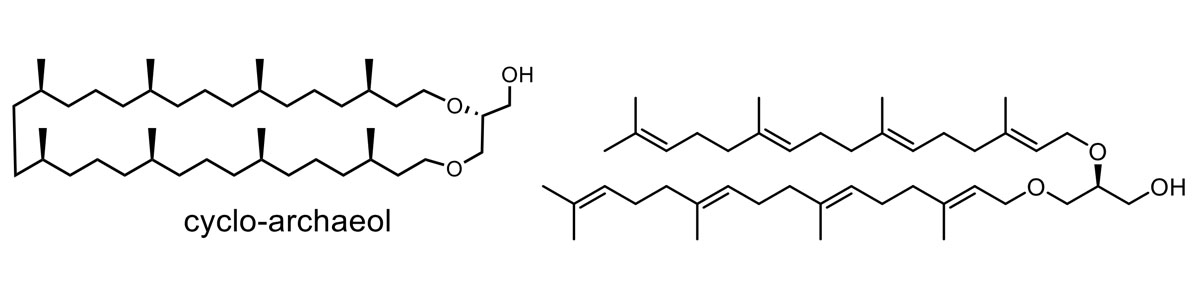
The synthesis and biosynthesis of Archaeal lipids
We are interested in the synthesis, biosynthesis and properties of membrane spanning lipids of archaea. This research is carried out in collaboration with the microbial physiology group of Prof. A.J.M. Driessen (UG).
Research Topic 4
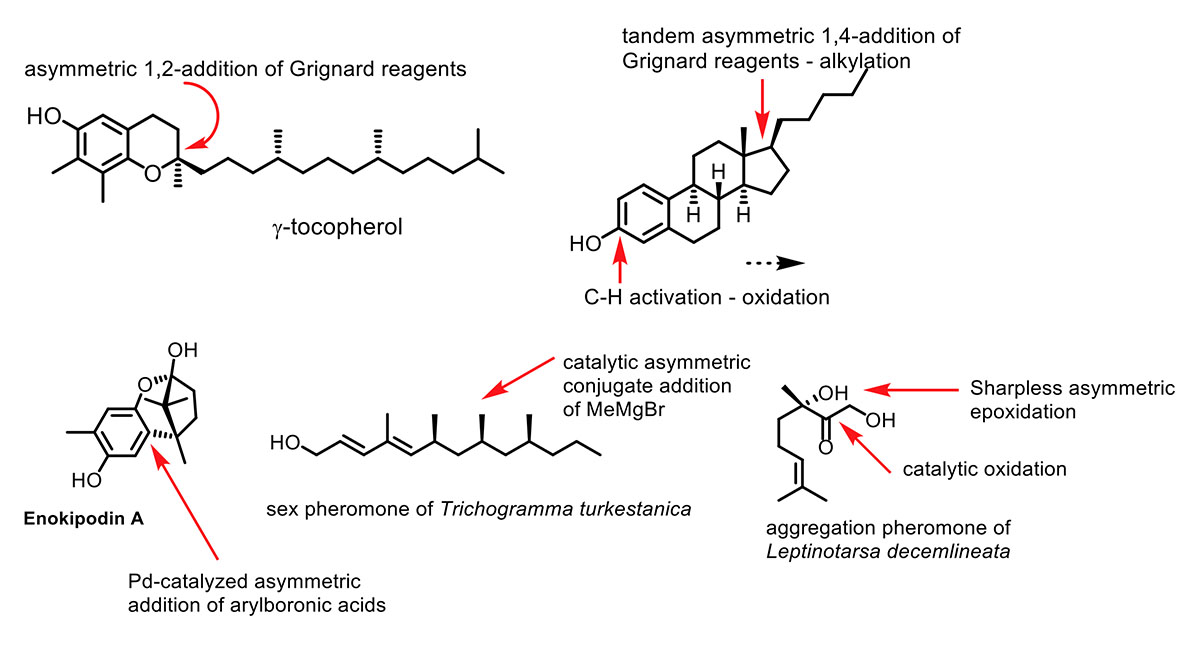
Asymmetric synthesis of quaternary stereocenters
The asymmetric synthesis of quaternary centers and tertiary alcohols and ethers is a formidable challenge. We developed together with the group of Prof. Harutyunyan, also within the Stratingh Institute, the first copper-catalyzed asymmetric addition of Grignard reagents to ketones in order to arrive at tertiary alcohols. We also develop palladium catalysts for the asymmetric conjugate addition of arylboronic acids to enones and this knowledge is used in natural product synthesis.